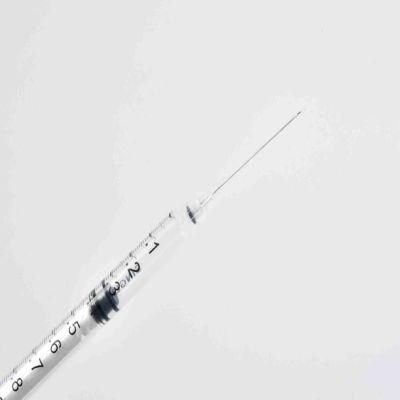
Medical Supply of Disposable Retractable Safety Syringe with ISO13485-2016/CE/Anvisa/FDA/Kgmp/Cfda
Maidikang Medical Products(Shandong) Co., Ltd.- Type:Surgical Supplies Materials
- Material:Medical PP
- Ethylene Oxide Sterilization:Eto Sterilization
- Quality Guarantee Period:5 Years
- Group:All
- Logo Printing:with or Without Logo
Base Info
- Model NO.:0.3,0.5,1,3,5ml
- Transport Package:Cartons
- Specification:0.3ml, 0.5ml, 1ml, 3ml, 5ml
- Origin:China
- HS Code:90183900
- Production Capacity:One Billion ,Year
Description

Using Manual
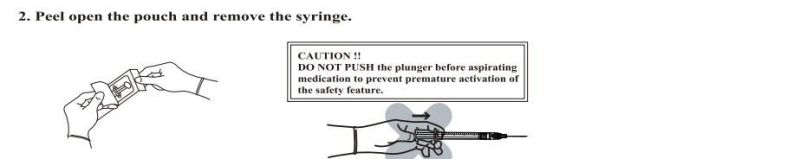
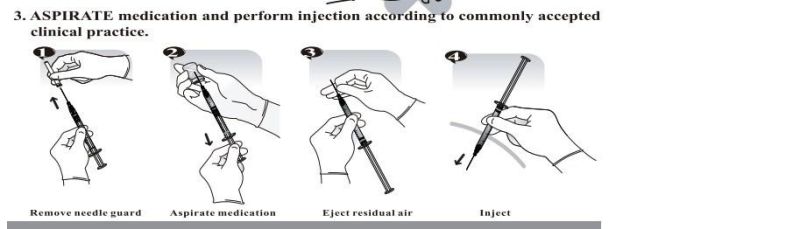
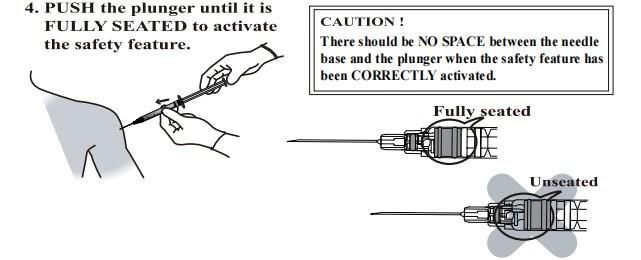
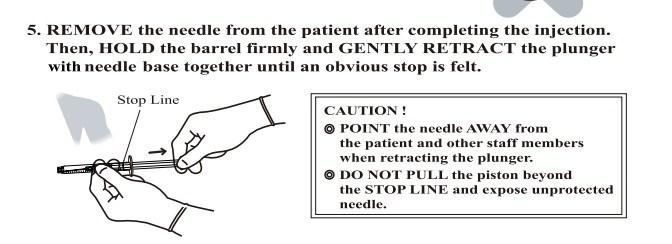
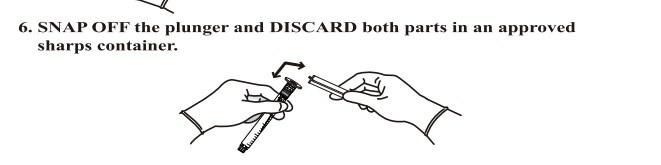
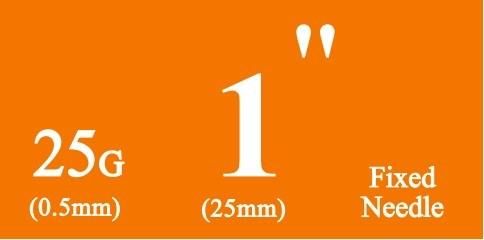
- Anticipate Usage: injecting medicine liquid to human body;
- Warning, Note and Presentation
- The medical device is only used for injecting medicine liquid to human body;
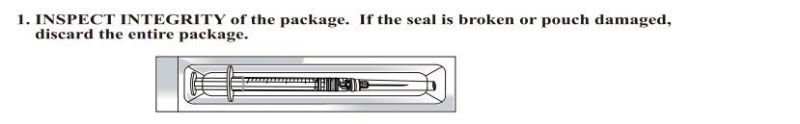
- If damaged package or unknown objects in it, it can't be used;
- The medical device can only single use, and destroy it after single use;
- The medical device is sterilized by EO, and it can't be used if expired validation;
- Validation: within 5 years after sterilization;
Conform to ISO 786-1 and ISO 7864
In compliance with European Medical Device Directive 93/42 EECE ( CE Class with needle IIa without needle I)
Quality Assurance
Manufacuring process is in compliance with ISO 13485 and IS09001 Quality systerm
Main material
PP SUS304 stainless Steel Cannula isoprene rubber silicone oil.
Certificate:CE ISO FDA 510K
COMPANY PROFILE
Nanchang Maidikang Medical Instrutment which started to produce syringe from 1990, we have more than 30 years in making medical products.
As a leading medical device company in China , we are dedicated to the researching, developing and producing of injection products.
Through the ISO 9001 quality system , FDA ,510K ,ANVISA,KGMP and CE product certification, the output one year is several billion pieces and can provide fast delivery for customers.
Our products and services include:
1 disposable insuline syringe:2 disposable syringes 1-50 ml luer lock or luer slip, with needle /safety needle or without needle3 three-way stopcocks4 auto-disable syringes5 disposable infusion sets6 hypodermic needles7 IV catheters8 surgical blades9 feeding tubes10 stomach tubes11 suction catheters12 disposable safety auto-disable syringes13 disposable safety hypodermic needles14 scalp vein sets15 disposable safety vacuum blood collection needles16 disposable vacuum blood collection needles17 disposable safety scalp vein sets18 retractable safety syringes( no gap type)19 safety vacuum blood collection needles( pen type)20 disposable syringes with safety needles21 disposable safety insulin syringes22 safety lancets23 disposable surgical proceture kits( masks, medical gauze applicator, medical tape sisposable syringe infusion sets)24 disposable urine bags25 surgical brushes26 filter needles for single use27 pre- filled flush syringes28 disposable feeding syringes and other medical products etc
29 OEM is also welcomed
We are committed to public health needs, by listening to customers' request and services, understand the needs of customers better , and this helps us to improve our existing products and develop new products.
Welcome to contact us any time and hope to cooperate if any support we can do for you.
FACTORY SHOW:

 ***************** YOU SAFETY ****** OUR CARE*****************
***************** YOU SAFETY ****** OUR CARE*****************FAQ
Q:Can we get free samples?
A: 1-10 pcs samples will be free , but the courier cost need to be on customers account.
Q: How about the delivery time ?
A: Between 5days to 30days depending on the quantity ordered. If you are sourcing a product, we will give you specific information regarding the lead time. If you need a rush order, just contact us to discuss your specific needs.
Q: How about the Minimum Order Quantity (MOQ) ?
A: For the first order, normally, the MOQ of syringe is 100,000 pieces
Q: Can you please quote me the C&F or CIF price?
A: In general, our quotation is based on FOB price. If you are looking for the C&F or CIF price then please inform your destination seaport and estimated quantity, we will quote you accordingly. Or you can recommend your shipping company to re-check the freight cost .
Q: What kind of certificate do you have ?
A: CE , ISO , FDA 510k, and other domestic certifications.
