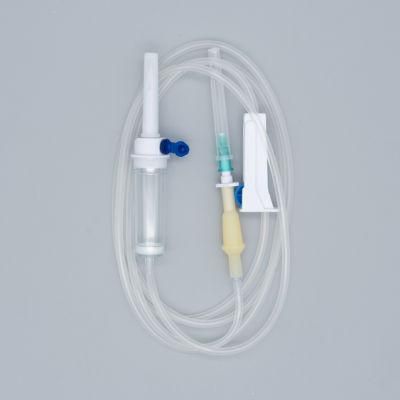
Qinkai Medical Quality CE Certified Disposable Infusion Set
Shandong Qinkai Medical Industry Co., Ltd.- Type:Infusion Set
- Material:PVC
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:QK-03
- Transport Package:PE
- Specification:61*38*32.5
- Trademark:Qinkai
- Origin:Heze
- HS Code:9018390000
- Production Capacity:300000pieces,Every Day
Description
Basic Info.
Model NO. QK-03 Transport Package PE Specification 61*38*32.5 Trademark Qinkai Origin Heze HS Code 9018390000 Production Capacity 300000pieces/Every DayProduct Description
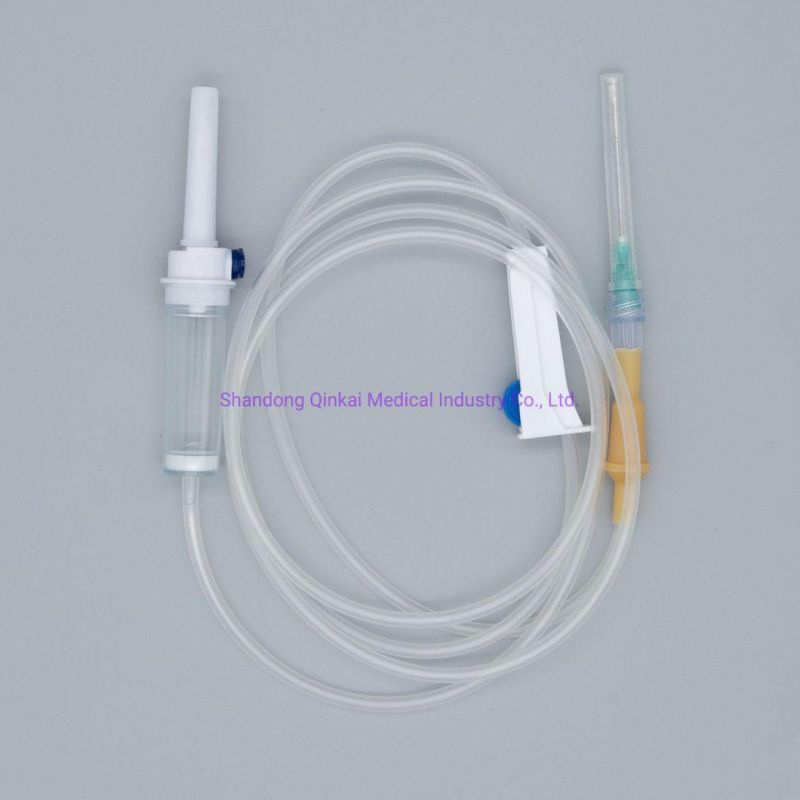
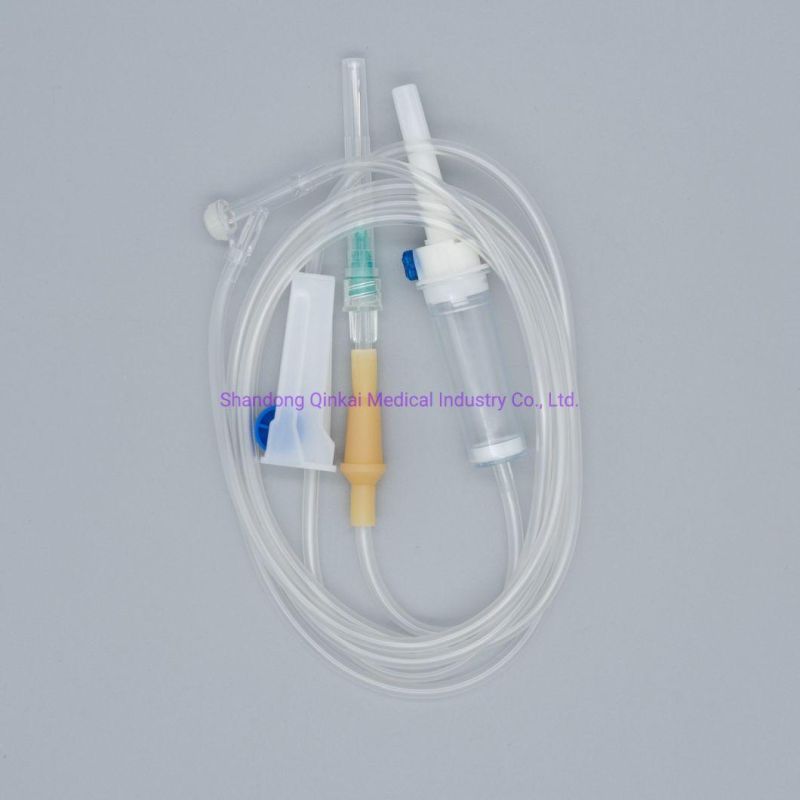
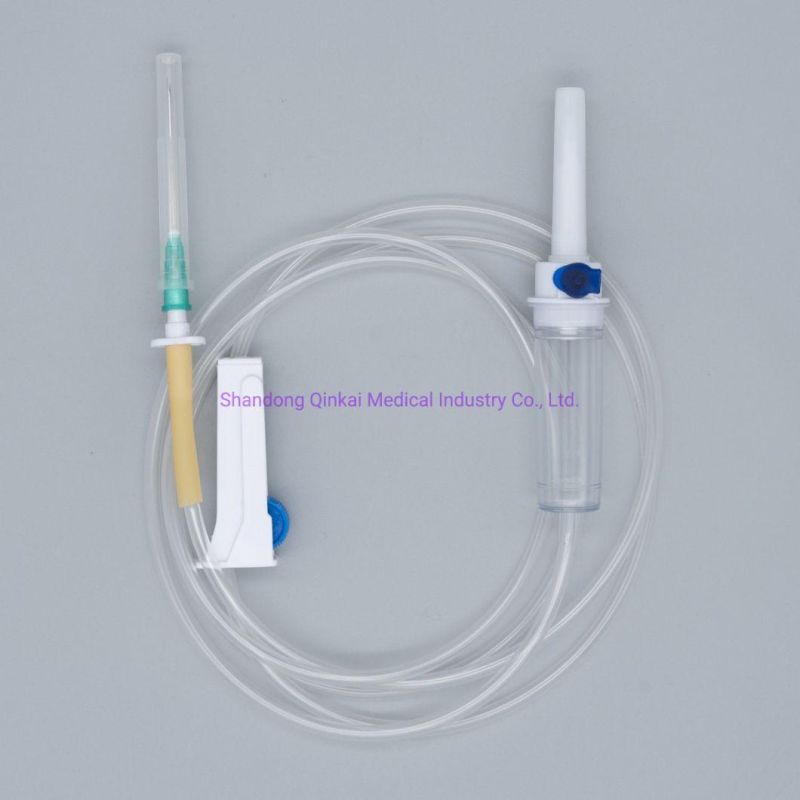

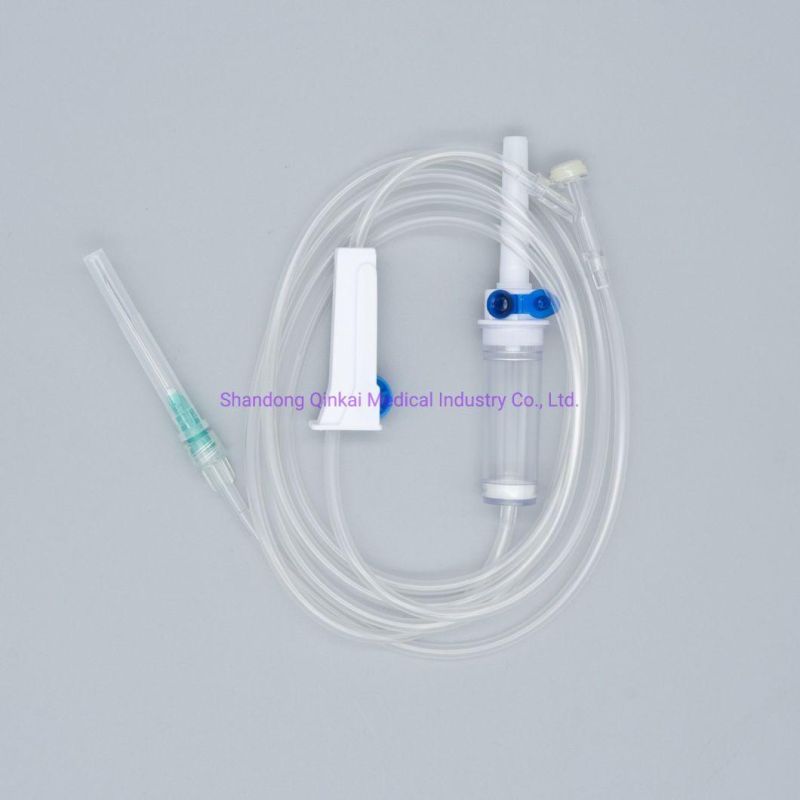
Product Description
Features:
1) Product structure: plastic spike for burette I.V sets ,with airvent , standard tube,
100ml chamber with floating valve ,common ABS flow regulator , "Y" connector, with two clamps ,luer slip without needle . Soft and thick tube
2) During infusion , the product is made by medical soft materials ,surface is sound
And transparent, soft and kink resistant medical grade PVC tubing .
5) scope for use : for intravenous gravity infusion
Reference standard : ISO8536-4
Options as customer's requiment
1.with or without air vented spike ;
2.with "Y" injection port or flash bulb injection port or without injection part
3. with or without needle
4. luer slip or luer lock
5. packed in PE or blister or complex transparent bag with paper
On-time Delivery
Company Information
Our factory is an enterprise certified by State Food & Drug Administration and specializing manufacturing sterile medical devices for single use.
Our factory was established in 1987 and covers an area of 240,000 square meters, including 100,000 class clean room which covers an area
of 42,000 square meters until now.
We have manufactured surgical gloves since 2005 and there are 50000pairs surgical gloves output each day, we have shared 30% local
market and exported surgical gloves to more than 60 countries.
We have quality control department to check the quality during raw materials, manufacturing, packaging, sterilizing and storage process.
We attend Medica in Dusseldolf, German and Mocow, Russia each year
Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
