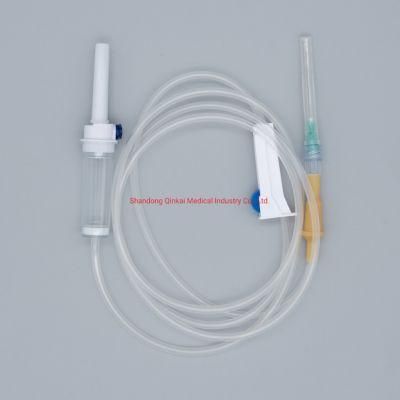
IV Infusion Set with Ce & ISO & FDA Approved
Shandong Qinkai Medical Industry Co., Ltd.- Type:Infusion Set
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
- Model NO.:qk-19
- Transport Package:PE
- Specification:61*38*32.5cm
- Trademark:qinkai
- Origin:China. Heze
- HS Code:9018390000
- Production Capacity:300000pieces,Every Day
Description
Basic Info.
Model NO. qk-19 Transport Package PE Specification 61*38*32.5cm Trademark qinkai Origin China. Heze HS Code 9018390000 Production Capacity 300000pieces/Every DayProduct Description
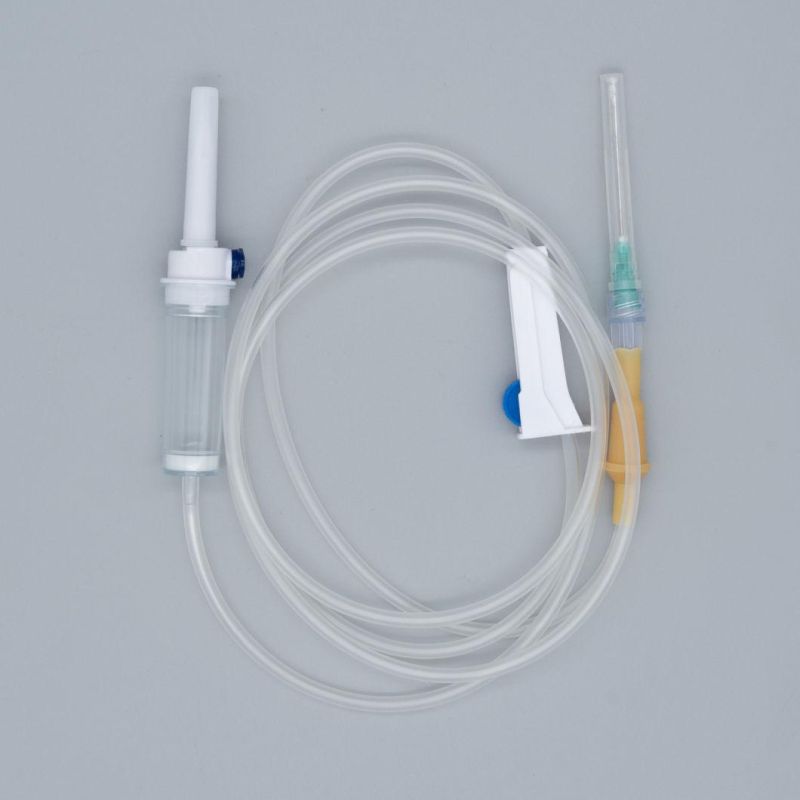
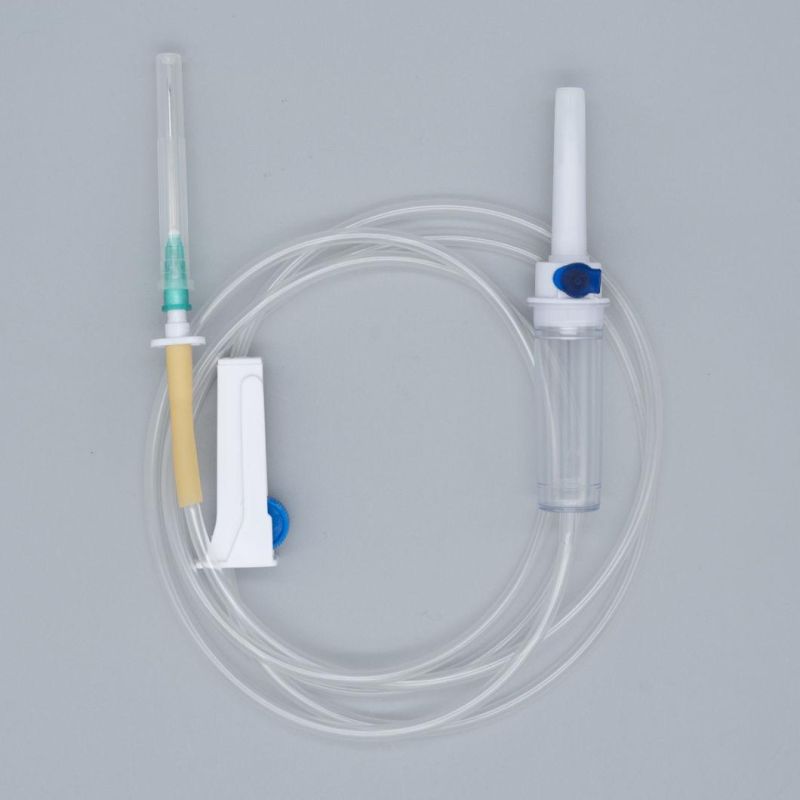
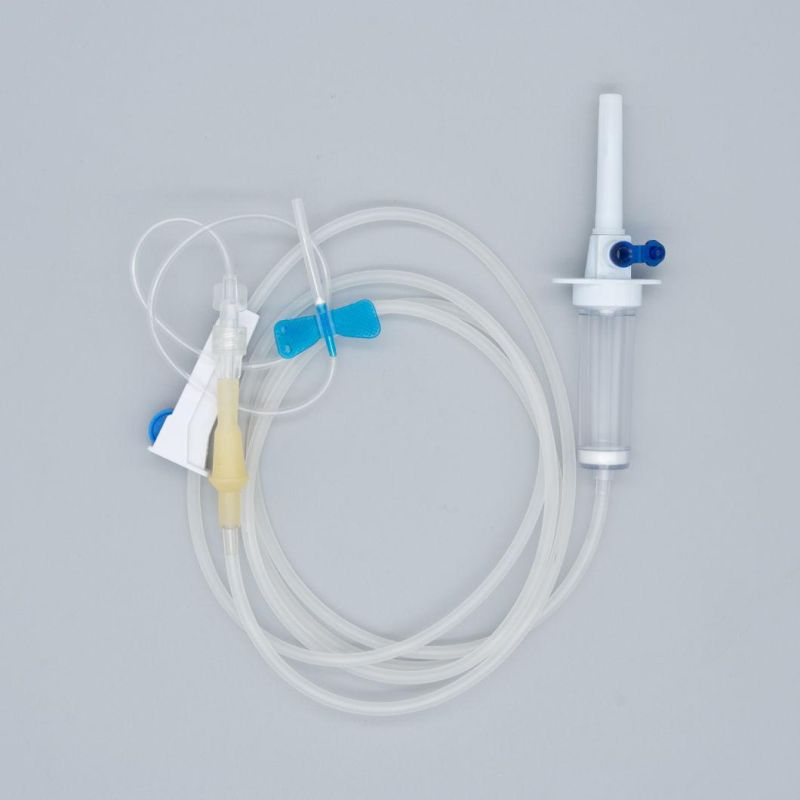
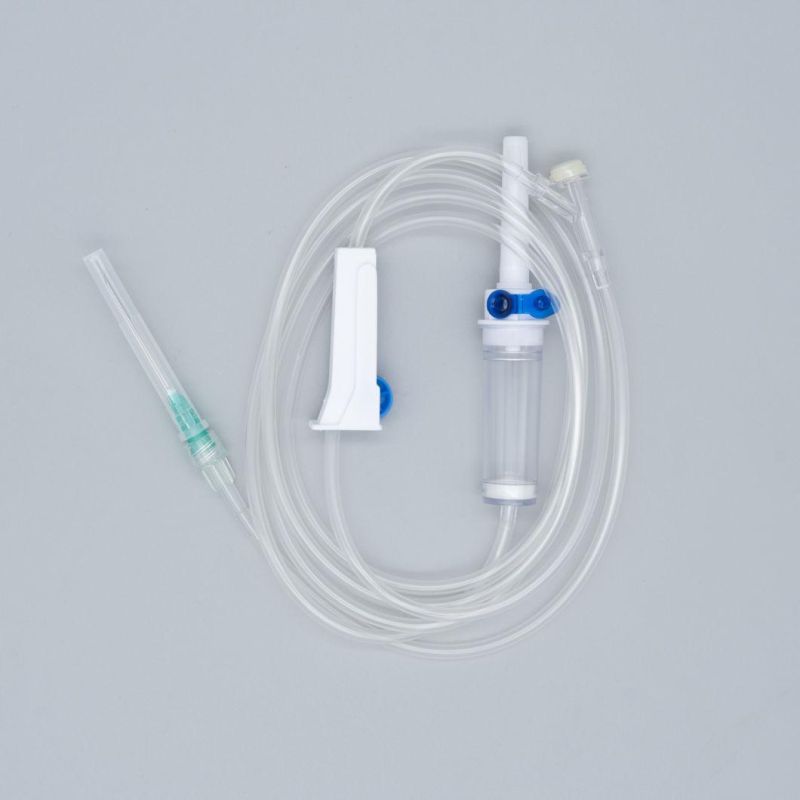 OverviewQuick DetailsProperties:Injection & Puncture Instrument
OverviewQuick DetailsProperties:Injection & Puncture InstrumentPlace of Origin:China
Brand Name:Goldenwell
Model Number:JLY--IVS001
Instrument classification:Class III
Product name:IV Infusion Set with CE & ISO & FDA Approved
Parts:spike, drop chamber, filter, tube, needle etc.
Package:PE bag, paper-plastic bag or as requested
Material:material grade PVC, DEHP free
Application:intravenous infusion
Needle connector:luer lock and luer slip
Tube length:1.35m, 1.5m, 2m or customized
Sterile:EO gas
Shelf life:3 years
Certificate:CE & ISO & FDAPackaging & DeliveryPackaging Details
IV Infusion Set with CE & ISO & FDA Approved
PE or paper-plastic bag indiivdually packing, 500PCS/carton
Port
Any Port of China for IV Infusion Set with CE & ISO & FDA Approved
Lead Time :
| Quantity(Pieces) | 1 - 100000 | >100000 |
| Est. Time(days) | 25 | To be negotiated |
IV Infusion Set with CE & ISO & FDA Approved
Specifications:
1. Main accessories: Vented spike, Drip Chamber, Filter, flow regulator, latex tube, needle connector, and needle;
2. Protective cap for closure piercing device made of polyethylene with internal thread that prevents the bacteria from coming in, but allows the entrance of ETO gas. Apporoximately 15 drops/ml, 20 drops/ml
3. Drip chamber made of soft PVC;
4. Flow Regulator made of polyethylene.
5. Soft and kink resistant medical grade PVC tubing .
6. Terminal fitting protective cap (luer slip or Luer-lock adapter upon request) made of PVC or polystyrene, according to the ISO 594/1 and 594/2 standards.
7. Terminal fitting protective cap made of polyethylene.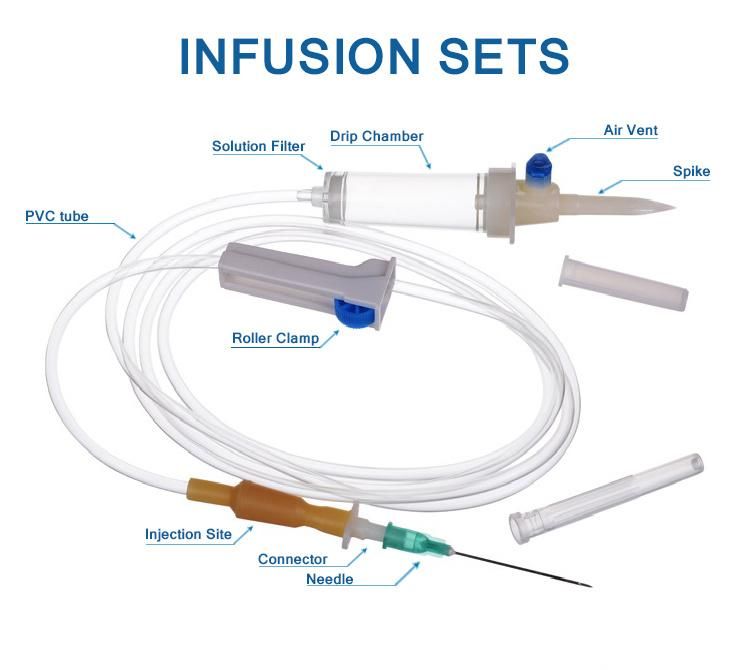
1. Why our price is the lowest?
Because we are a factory.
2, How about the lead time?
About 15 working days after receiving the payment and confirming all the artworks, exactly lead time upon the quantity of your order and the packaging you required.
3, Can our private logo / label be printed on the packaging?
Yes, your own private logo/ label can be printed on the packaging upon your legal authorization, we do OEM service for many years.
4.How can i get some samples?
1. We may provide some samples of free, the postage will be paid by yourself. The post charges will be deducted from payment for goods after we bargained on the order .
2. You can give us your collect account (just like DHL, UPS etc) and detail contact information. Then you can pay the freight direct to your local carrier company.
5. What is the best price you can offer?
We always working hard to satisfy our customer, from the quality until the price, as we do understand the market situation. So, please don't hesitate to send your inquiry for us to give you our best price.
6. Why choose us?
1. We are the leading medical devices manufacturer in China since 1988.
2. Alibaba verified factory, passed FDA & CE & ISO & GMP.
3. Best service and nice quality with competitive prices.
Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
