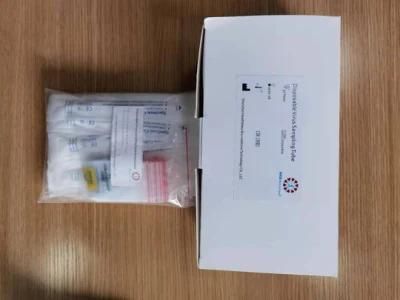
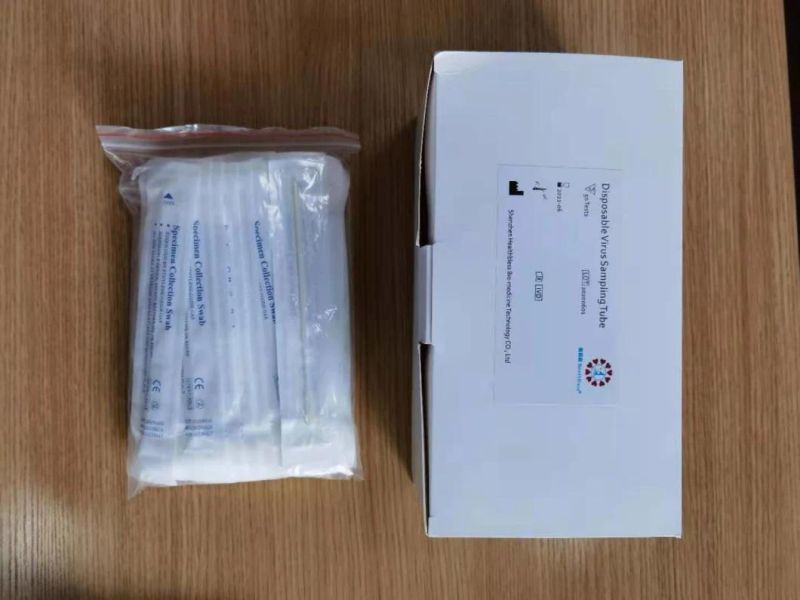
Virus Specimen Collection Kit Virus Sampling Tube with Sterile Swab Sticks
Anhui Yaliya Biotechnology Co., Ltd.- Type:Test Strips & Test Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Transport Package:Suit for The Exporting Packaging
- Specification:Type I: Inactivated including swab
Base Info
- Origin:China
- Production Capacity:10000000sets,Month
Description
Basic Info.
Origin China Production Capacity 10000000sets/MonthProduct Description




Quick Details
Place of Origin:
China
Brand Name:
HealthBless
Model Number:
Type I, Type II, Type III, Type IV
Disinfecting Type:
EOS
Properties:
Medical Materials & Accessories
Size:
Type I, Type II, Type III, Type IV
Stock:
yes
Shelf Life:
2 years
Material:
PP
Quality Certification:
CE,ISO13485
Instrument classification:
Class I
Safety standard:
MFDS
CERTIFICATE:
CE/ISO13485
Supply Ability
Supply Ability:
5000000 Set/Sets per Month
Packaging & Delivery
Packaging Details
SUIT FOR THE MEDICAL DEVICES PACKAGING AND STANDARD EXPORTING PACKAGING
Port
SHANGHAI PORT
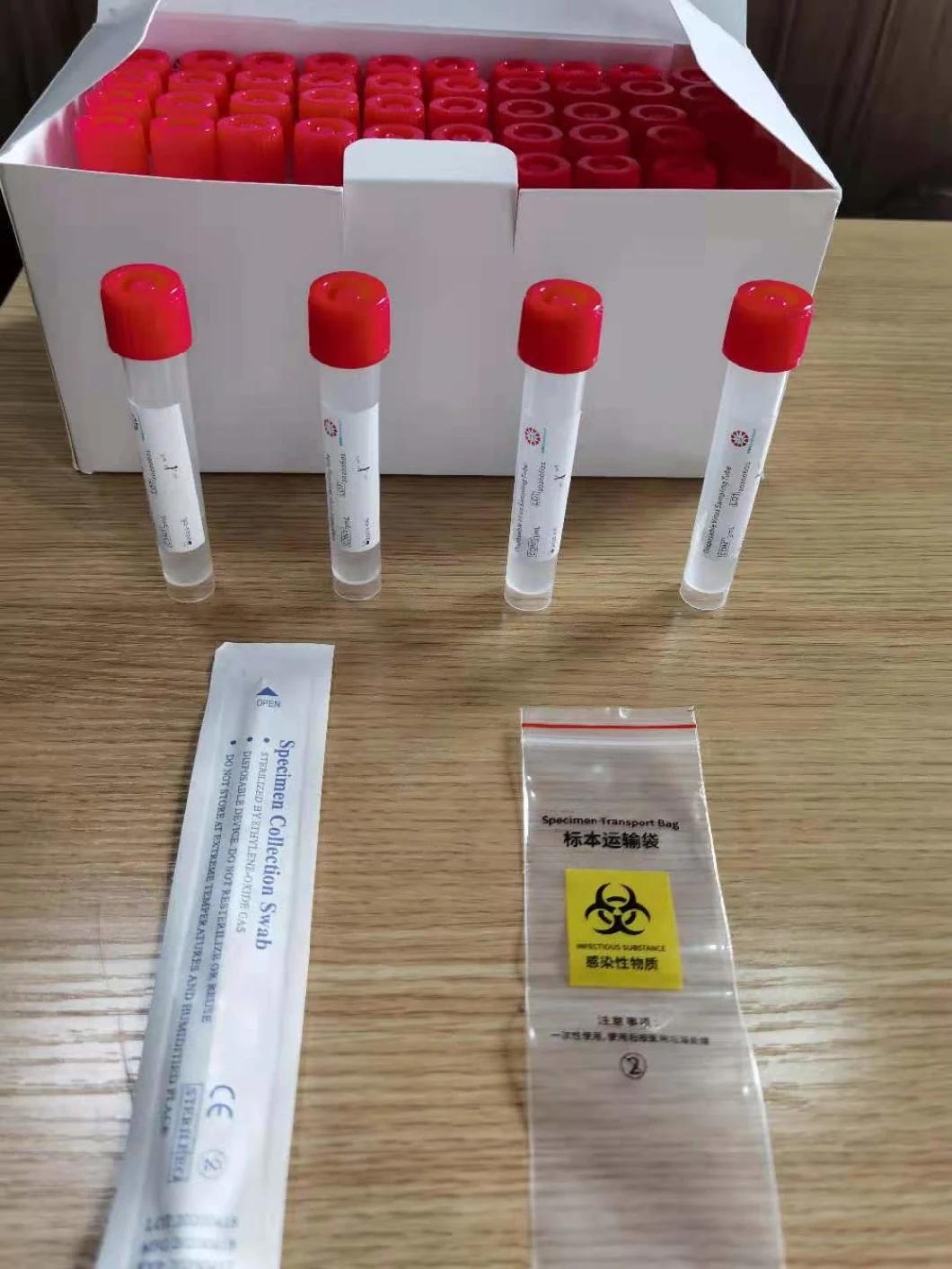
Disposable virus sampling tube
(Type I: Inactivated including swab)
Product record No.: GuangDong ShenZhen medical devices 20200187
Production record No,: GuangDong ShenZhen 20160030.
EU CE certification
ISO13485 system certificate
Exclusive non-guanidine inactivating activity, safety and environmental protection consideration!
Safety, stability, and multiple models
Can be exported in large quantities
Non-guanidine inactivating activity (exclusive nationwide)
Guanidine inactivating activity
Inactivated
Centers for Disease Control and Prevention
Infectious disease hospitals
Other Designated hospitals for the diagnosis and treatment
Essential products for Disease Control and Prevention
Safety, stability, multi-function
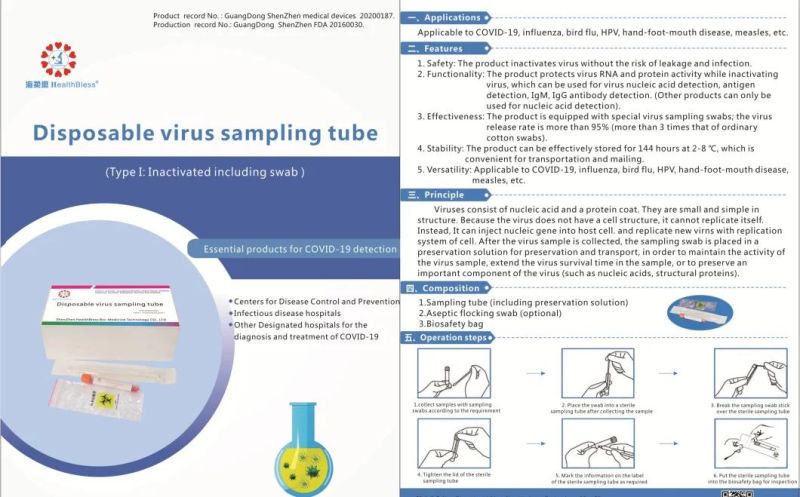
Applications
Applicable to influenza, bird flu, HPV, hand-foot-mouth disease, measles, etc.
Features
Safety: The product inactivates virus without the risk of leakage and infection.
Functionality: The product protects virus RNA and protein activity while inacti
Inactivating virus, which can be used for virus nucleic acid detection, antigen detection, IgM, IgG antibody detection. (Other products can only be used for nucleic acid detection).
3,Effectiveness: The product is equipped with special virus sampling swabs; the virus release rate is more than 95% (more than 3 times that of ordinary cotton swabs).
Stability: The product can be effectively stored for 144 hours at 2-8 °C, which is convenient for transportation and mailing.
Versatility: Applicable to influenza, bird flu, HPV, hand-foot-mouth disease,measles, etc.
Environmental protection: Non-guanidine inactivated preservation solution can effectively avoid environmental problems caused by the use of guanidine inactivated preservation solution.
Principle
Viruses consist of nucleic acid and a protein coat. They are small and simple in structure. Because the virus does not have a cell structure, it cannot replicate itself. Instead, It can inject nucleic gene into host cell, and replicate new virns with replication system of cell. After the virus sample is collected, the sampling swab is placed in a preservation solution for preservation and transport, in order to maintain the activity of the virus sample, extend the virus survival time in the sample, or to preserve an important component of the virus (such as nucleic acids, structural proteins).
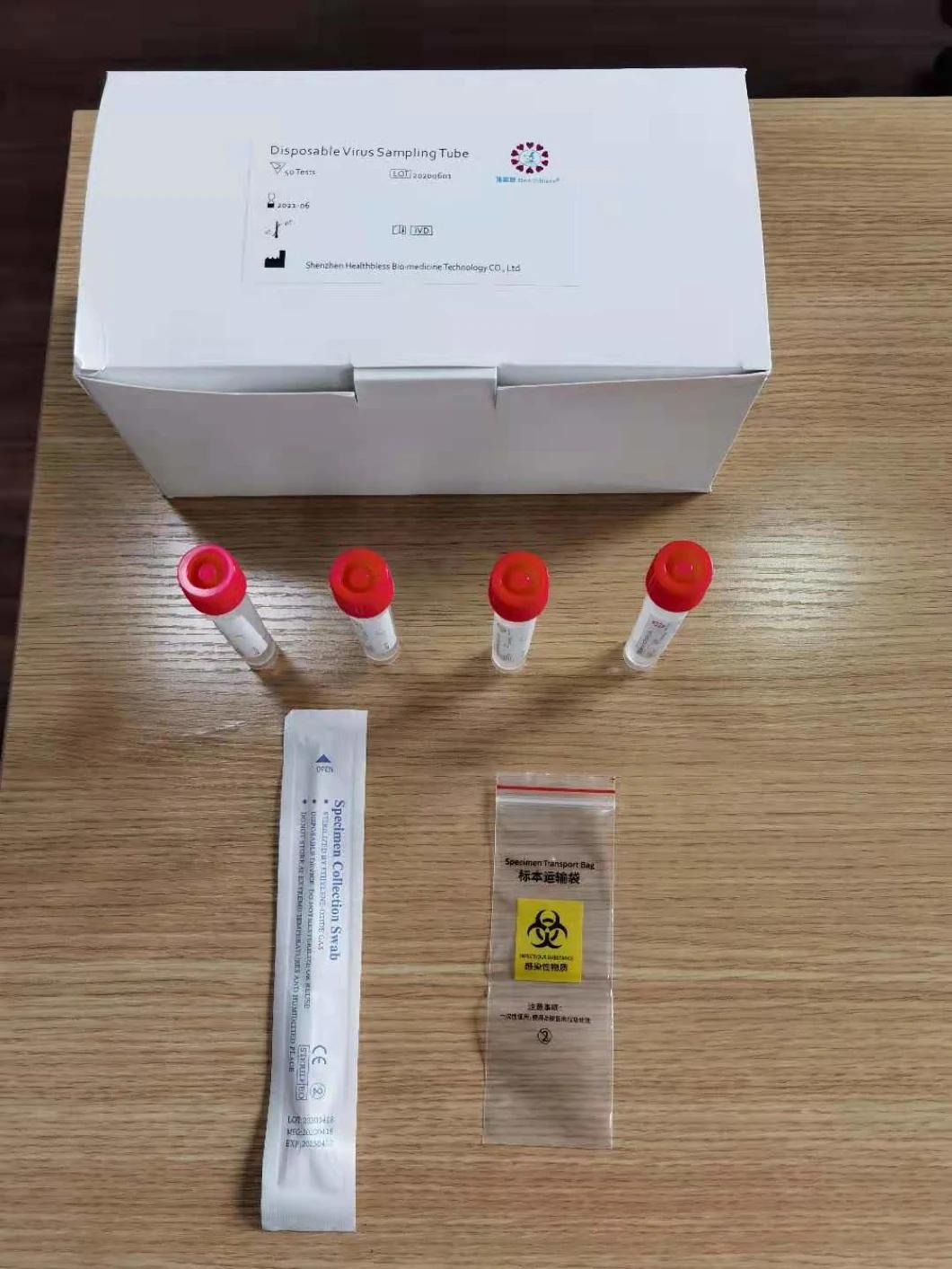
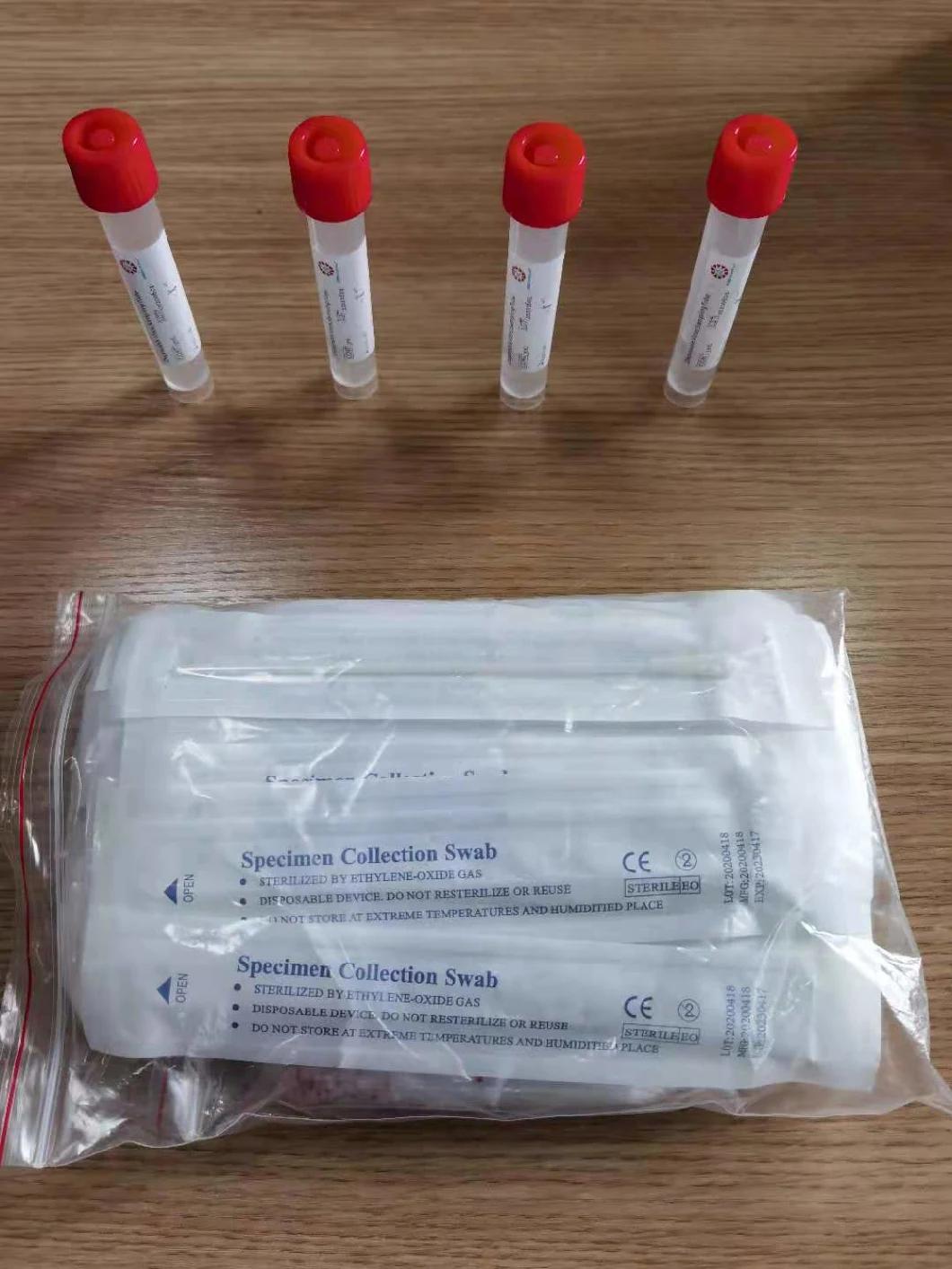
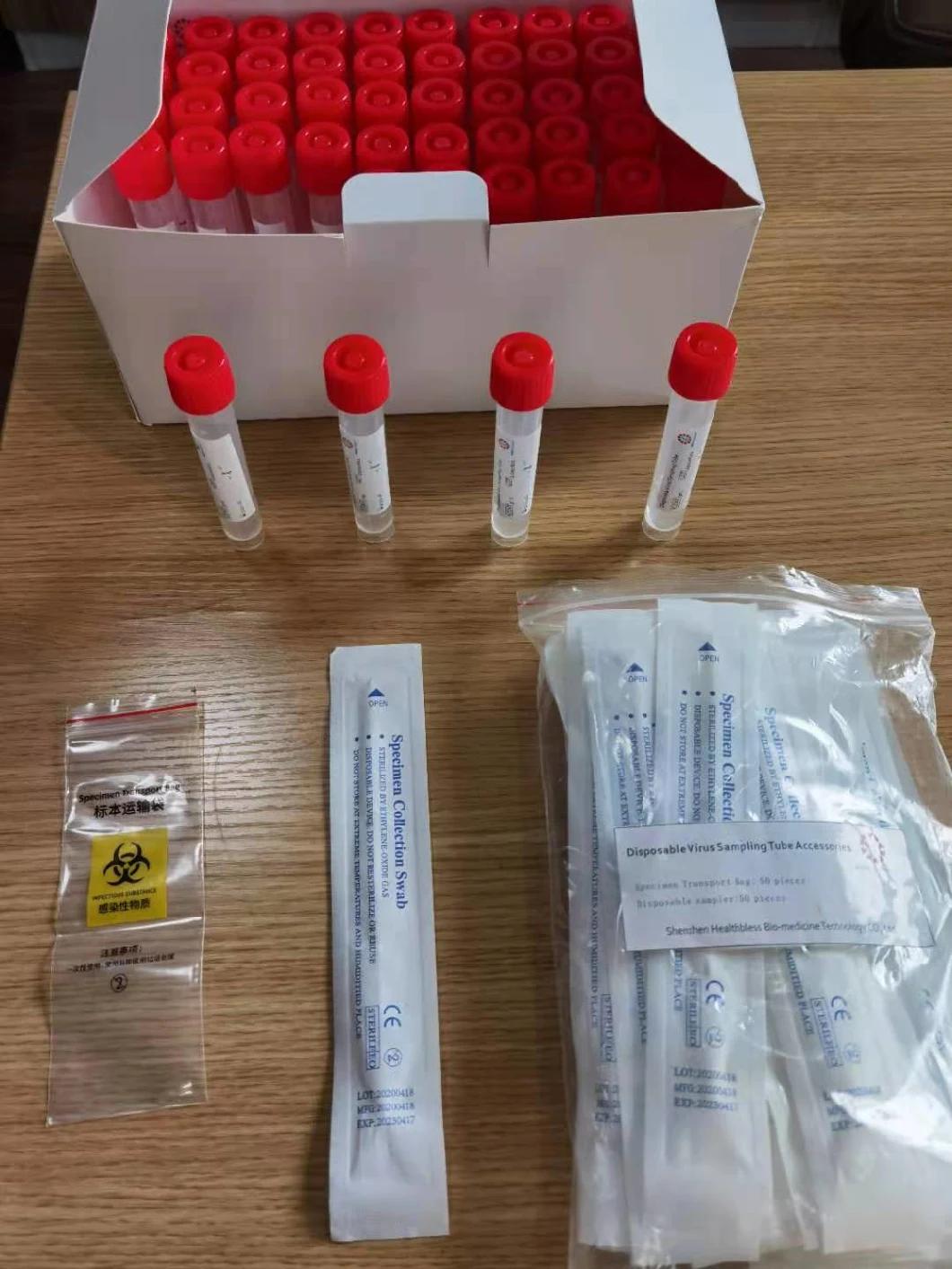
Composition
Sampling tube (including preservation solution)
Aseptic flocking swab (optional)
Biosafety bag
Operation steps
According to sampling requirements, the sample is collected with sampling swab
Place the swab into a sterile sampling tube after collecting the sample .
Break the sampling swab stick over the sterile sampling tube.
Tighten the lid of the sterile sampling tube
Mark the information on the label of the sterile sampling tube as required
Put the sterile sampling tube into the biosafety bag for inspection.
For more information please visit our website:
Contact person: Mr Kevin Chen
