
Guide Wires for The Treatment of Coronary Heart Disease
Suzhou Innomed Medical Device Co., Ltd.- Type:Surgical Supplies Materials
- Material:Nickeltitanium Wire Core
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:Inno-Pathwire
- Certification:CE,ISO13485
- Application:Ptca,Pta
- FDA Certificate Number:K182553
- Minimum Unit of Measurement:1 Set,Bag
- Transport Package:Cardboard Box
- Specification:IW-18-300-MDS
- Trademark:Innomed
- Origin:Suzhou
- HS Code:9018909919
- Production Capacity:50000,Y
Description
Cautions
1.This Inno-Pathwire should be used only by physicians trained in angiography and Percutaneous Transluminal Coronary Angioplasty (PTCA), and/or Percutaneous Transluminal Angioplasty (PTA).
2.Refer to the instructions supplied with the interventional devices to be used with the Inno-Pathwire for their intended uses, contraindications, and potential complications.
3.Do not use if the package is open or damaged.
4.Do not use if it is expired.
Device Description
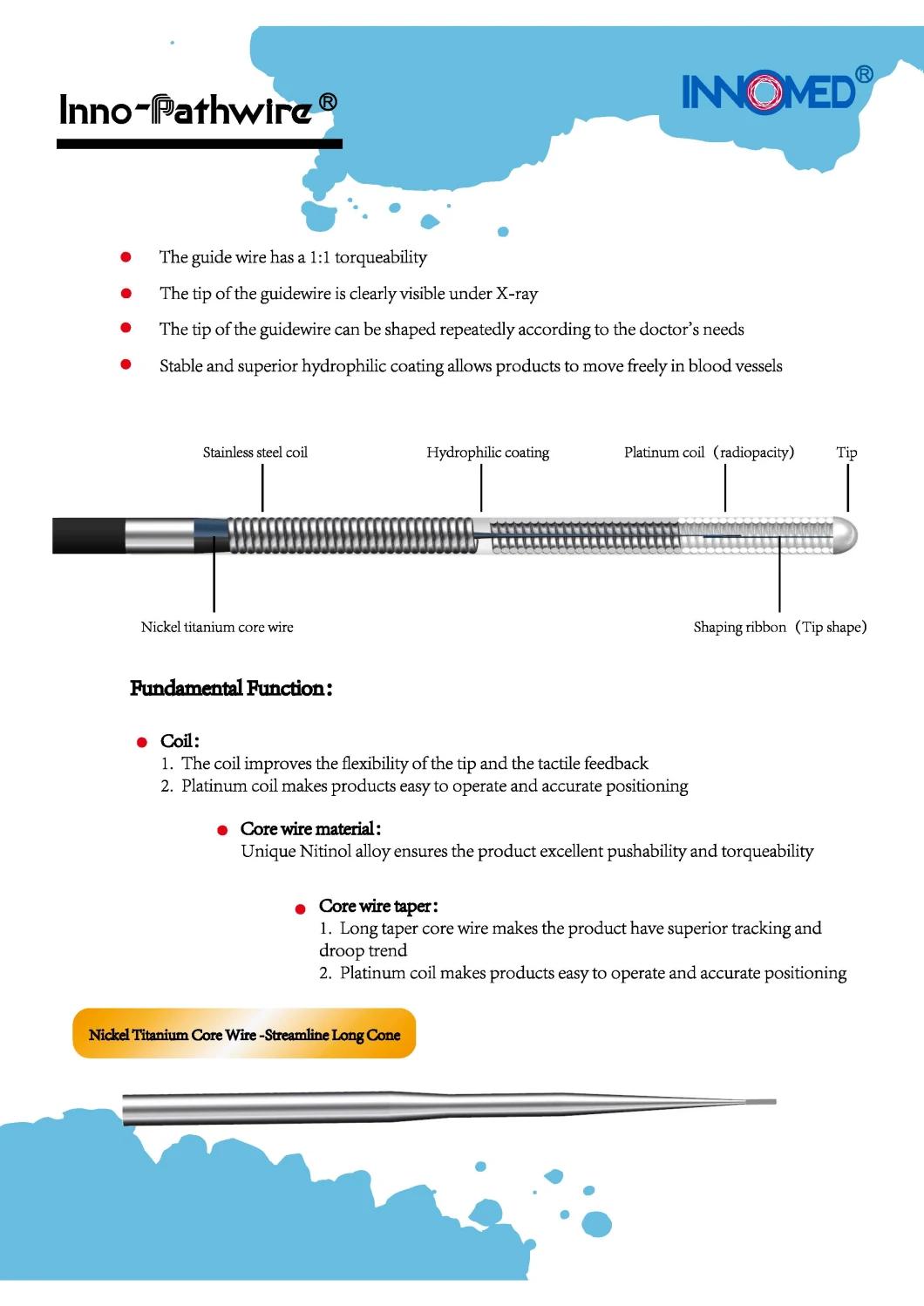
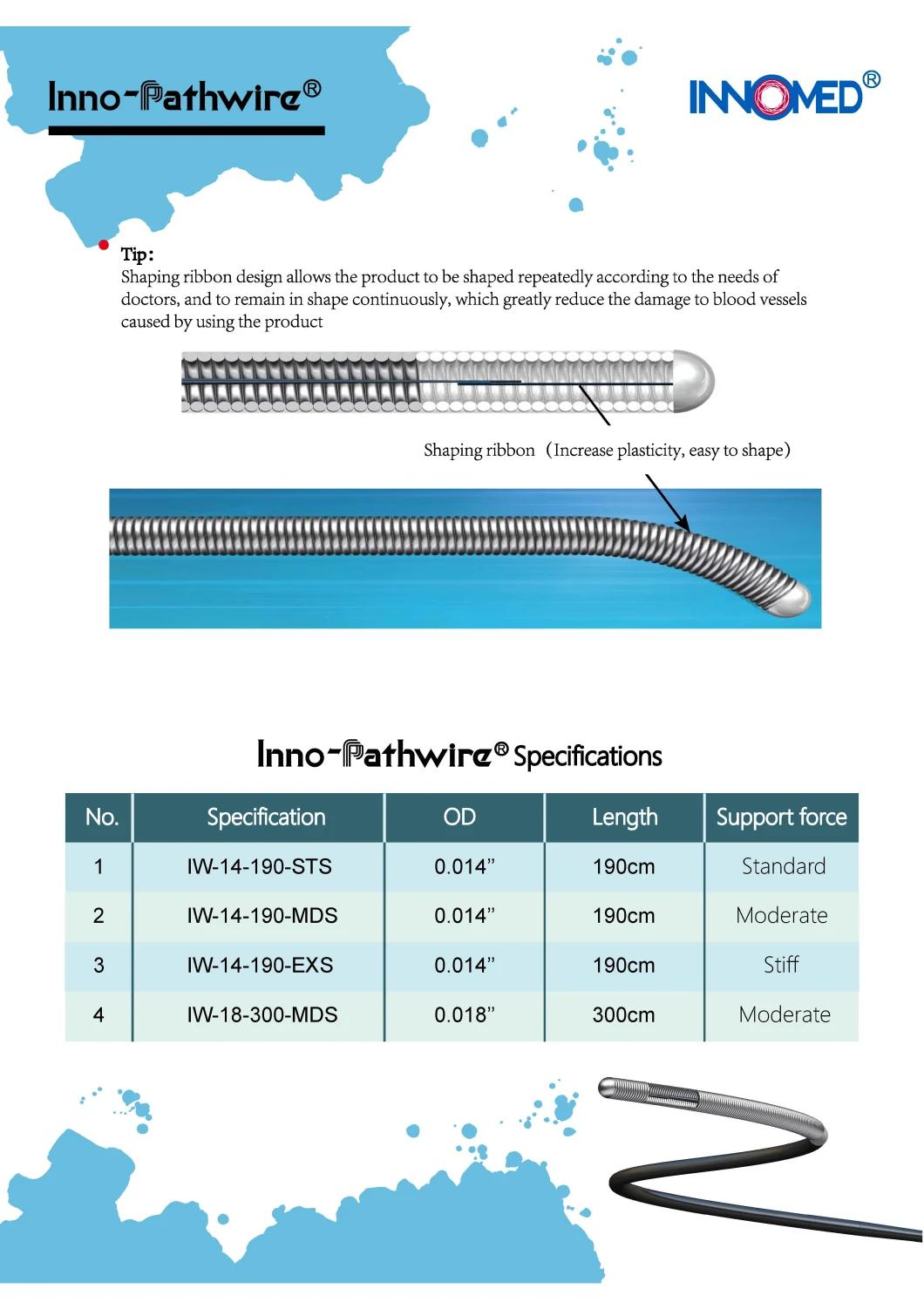
The Inno-Pathwire is a steerable guidewire available in a diameter of 0.014" (0.36 mm) and an effective length of 190 cm. The distal tip is shapeable. Before use, the tip can be shaped per standard technique. The distal tip is visible under X-ray to assist the physician with the position and movement of the guidewire. The distal segment of the guidewire, up to the hypotube, is coated with hydrophilic coating to reduce friction for improved guidewire movement within the catheter and arteries. The proximal end of the guidewire is coated with PTFE which reduces friction of the wire.
Device Specification
The Inno-Pathwire guidewires has three models. Refer to the product label for product specifications (e.g., wire length, diameter, length of tip radiopacity, and shaft stiffness).
Directions for use
1. Carefully insert the guidewire through the guidewire lumen hub of the interventional device.
2. Advance the guidewire until its tip is just proximal to the interventional device tip.
3. If using a guiding catheter, engage the guiding catheter and insert the interventional device/ guidewire assembly through the hemostatic valve. Advance the system through the guiding catheter until it is just proximal to the tip of the guiding catheter.
4. Tighten the hemostatic valve to create a seal around the interventional device. Ensure intentional guidewire movement is still permitted.
5. Attach the torque device to the guidewire, if desired.
6. Under fluoroscopy, advance the guidewire out of the interventional device while securing the interventional device in place. Use the torque device to steer the guidewire across the lesion.
7. Secure the guidewire in place while tracking the interventional device over it and to the target lesion.
8. If a different tip configuration or guidewire is indicated, carefully remove the guidewire while observing guidewire movement under fluoroscopy.
9. Reshape the guidewire tip according to standard practice or prepare another guidewire to be used.
10. Reinsert the guidewire following Steps 1 through 7 of this section.
Product Line



Certificate



