-
High Quality OEM Logo Printed Tie on Face Mask
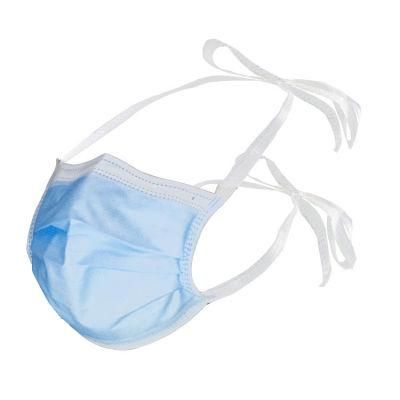
Overview Product DescriptionDetailed PhotosProtective LevelCertificationsCompany ProfileOur FactoryProduct PackagingFAQ1. who are we?We are based in Hubei, China, start from 2006,sell to Eastern Europe(40.00%),Mid East(27.00%),Domestic Market(12.00%),North America(11.00%),Central
-
3D Comfortable 3 Ply Ear Loop Face Mask

1.3-ply defend, cleaner and safer2.Ear-loop /tie-on, more choices3.Nose clip contours to avariety of face shapes and sizes4.Ultrasonically sealed soft stretched "latex face" earloops, safer and cleaner5.Packing:50pcs/box, 2000pcs/carton, ctn size:52*38*50cm 20GP=580000pcs, 40GP=1220000PCS,
-
Disposable Isolation Clothing Surgical Gown Isolation Gown

Product DescriptionNameHospital disposable medical surgical isolation gown sms pp pe non woven isolation gownMaterialNon-woven Fabrics (PP/SMS/PE)StyleWith tie on back neck and waist, elastic/ knitted cuff, round neck, long/ middle/ short sleeveScope of applicationMedical & health / household /
-
3ply Disposable Medical Surgical Black Face Mask

Overview Product DescriptionDetailed PhotosCompany ProfileHubei Wanli Protective Products Co.,ltdPackaging & Shipping
-
Cotton Kinesiology Tape Hypoallergenic Kinesiology Tape Tape

Overview Product ParametersDetailed PhotosCompany ProfileFAQQ1: Are you a manufacturer of MedicalConsumables?A: Yes, we are a professional manufacturer of Household&Medical Consumables with 20 years' experience. Offering various size of products with good quality and competiti
-
Cotton Kinesiology Tape Recovery Sports Muscle Tape

Overview Product ParametersDetailed PhotosCompany ProfileFAQQ1: Are you a manufacturer of MedicalConsumables?A: Yes, we are a professional manufacturer of Household&Medical Consumables with 20 years' experience. Offering various size of products with good quality and competiti
-
Medical Cotton Elastic Kinesiology Tape for High Performances

Overview Product ParametersDetailed PhotosCompany ProfileFAQQ1: Are you a manufacturer of MedicalConsumables?A: Yes, we are a professional manufacturer of Household&Medical Consumables with 20 years' experience. Offering various size of products with good quality and competiti
-
Hospital Medical Disposable Non-Woven Protective Surgical Gown

Product DescriptionNameHospital disposable medical surgical isolation gown sms pp pe non woven isolation gownMaterialNon-woven Fabrics (PP/SMS/PE)StyleWith tie on back neck and waist, elastic/ knitted cuff, round neck, long/ middle/ short sleeveScope of applicationMedical & health / household /


 主站蜘蛛池模板:
贺兰县|
思南县|
大庆市|
察雅县|
壤塘县|
马鞍山市|
砀山县|
剑川县|
平乡县|
比如县|
龙游县|
綦江县|
曲周县|
苏尼特右旗|
西藏|
扎鲁特旗|
无为县|
静安区|
张家港市|
郎溪县|
郯城县|
临安市|
阿拉善右旗|
无棣县|
开鲁县|
连山|
尼木县|
禹州市|
台中市|
凤冈县|
句容市|
区。|
太和县|
射洪县|
瓦房店市|
隆尧县|
太康县|
蓬安县|
塔城市|
武强县|
芜湖市|
主站蜘蛛池模板:
贺兰县|
思南县|
大庆市|
察雅县|
壤塘县|
马鞍山市|
砀山县|
剑川县|
平乡县|
比如县|
龙游县|
綦江县|
曲周县|
苏尼特右旗|
西藏|
扎鲁特旗|
无为县|
静安区|
张家港市|
郎溪县|
郯城县|
临安市|
阿拉善右旗|
无棣县|
开鲁县|
连山|
尼木县|
禹州市|
台中市|
凤冈县|
句容市|
区。|
太和县|
射洪县|
瓦房店市|
隆尧县|
太康县|
蓬安县|
塔城市|
武强县|
芜湖市|



