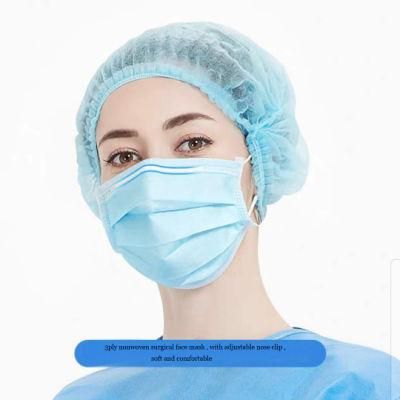
3ply Nonwoven White List Manufacturer Surgical Face Mask Sud TUV Report
Zhejiang Yinda Biotechnology Co., Ltd.- Type:Face Mask,Apron,Gloves
- Material:Non-woven Fabric
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
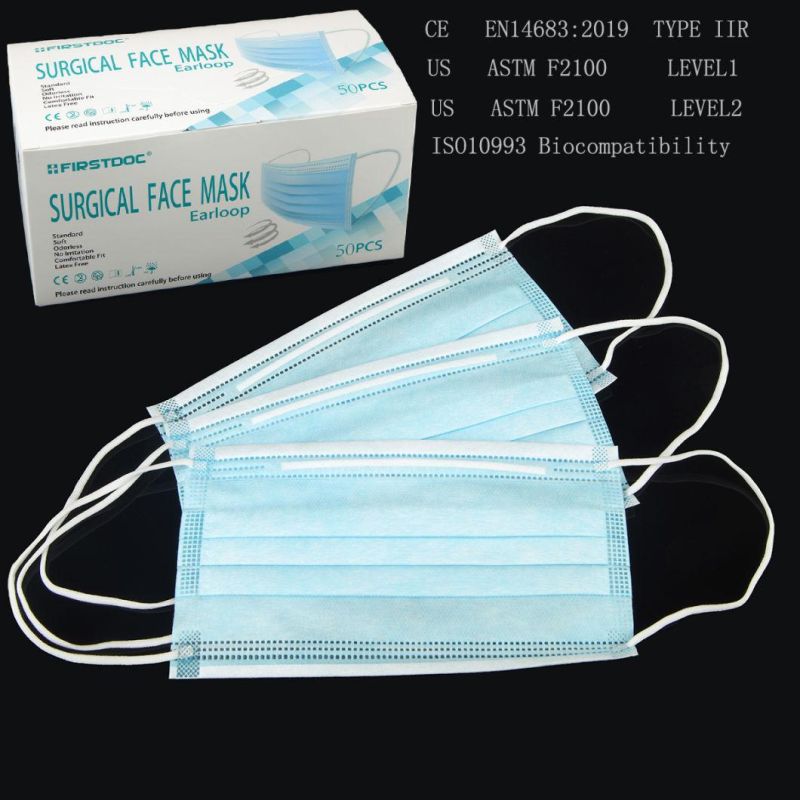
- Model NO.:YD-WKA
- Color:White, Blue, Black, Pink
- Transport Package:Carton
- Specification:9.5CM*17.5CM
- Trademark:FIRSTDOC
- Origin:China
- HS Code:6307900010
- Production Capacity:500000PCS,Day
Description
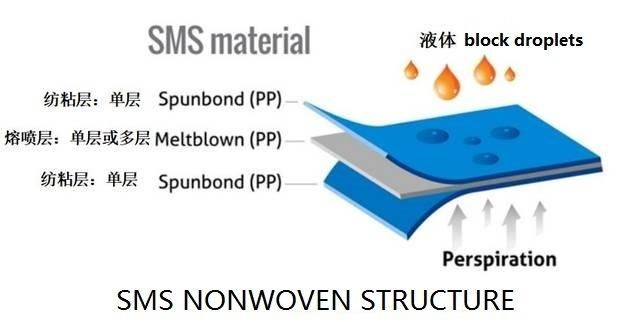
1st ply:25-28g non-woven ,
2ply :meltblown filter paper
3ply: 25-28g non-woven
Earloop : polyester+ spandex
Nose Clip :pp
Carton weight :4KGS±0.2KGS
Usage: Surgical face mask is intended for single use for operating room personnel and other general healthcare works to protect both patients and healthcare works against transfer of microorganism , blood and body fluid and particulate materials .
How to use :

Restriction: 1 This product produce oxygen , and can no longer be used in anoxic environment
2 Donot use face mask when facial beard or others can affect the tightness between face and mask ;3 the best continuous use time is 4 hours ;4 Patient with dyspnea diseases such as asthma are not allowed to use
Storage : Store in cool , dry ,well ventilated place away from direct sunlight .

Company Introduction :
Zhejiang Yinda Biotechnology Co.,Ltd , located in Dongyang Economic Development Zone , was founded in 1999 , which is a professional manufacturer producing class I , class II medical device , face mask , cooling gel patch ,pain relief patch , adhesive bandage , nasal strip , first aid kit , etc. cover an area of 12000M2 , factory plant 26000M2 ,with more than 200 staffs , 100.000 level GMP purification workshop , 10.000 level detection research and development center , we pass ISO9001:2008, FDA , CE , ISO13485 certificate , "excellence , development , innovation and honest" is our goal , we are looking forward to your enquiries and establish cooperation in long time .




If you have any questions, please contact me.
I am of your service at any time.
